
Math and Science Team ក្រុមអ្នកគណិតវិទ្យា និងវិទ្យាសាស្រ្ដ - Lectured by YIM VANNAK ( chemistry and Laboratory Management ) pH, pOH, pKa, and pKb The symbol "p" means "the negative of the logrithm of." "

pKa & pH Values| Functional Groups, Acidity & Base Structures - Video & Lesson Transcript | Study.com

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
![SOLVED: Question #16 In buffer calculations, we used Henderson Hasselbalch equation. [A-]" pH pKa + log1o THA Suppose you are using a weak monobasic acid, HA, with a pKa of 4.0. Suppose SOLVED: Question #16 In buffer calculations, we used Henderson Hasselbalch equation. [A-]" pH pKa + log1o THA Suppose you are using a weak monobasic acid, HA, with a pKa of 4.0. Suppose](https://cdn.numerade.com/ask_previews/3d97130d-3610-4ac7-8aee-a2500b0f71f3_large.jpg)
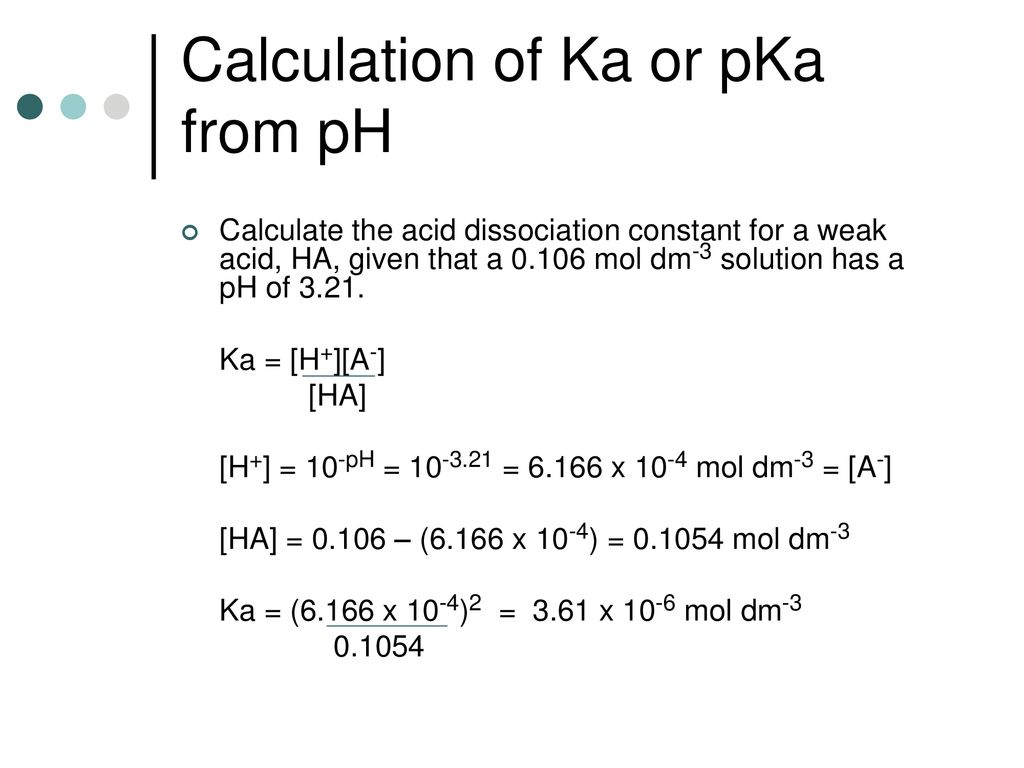
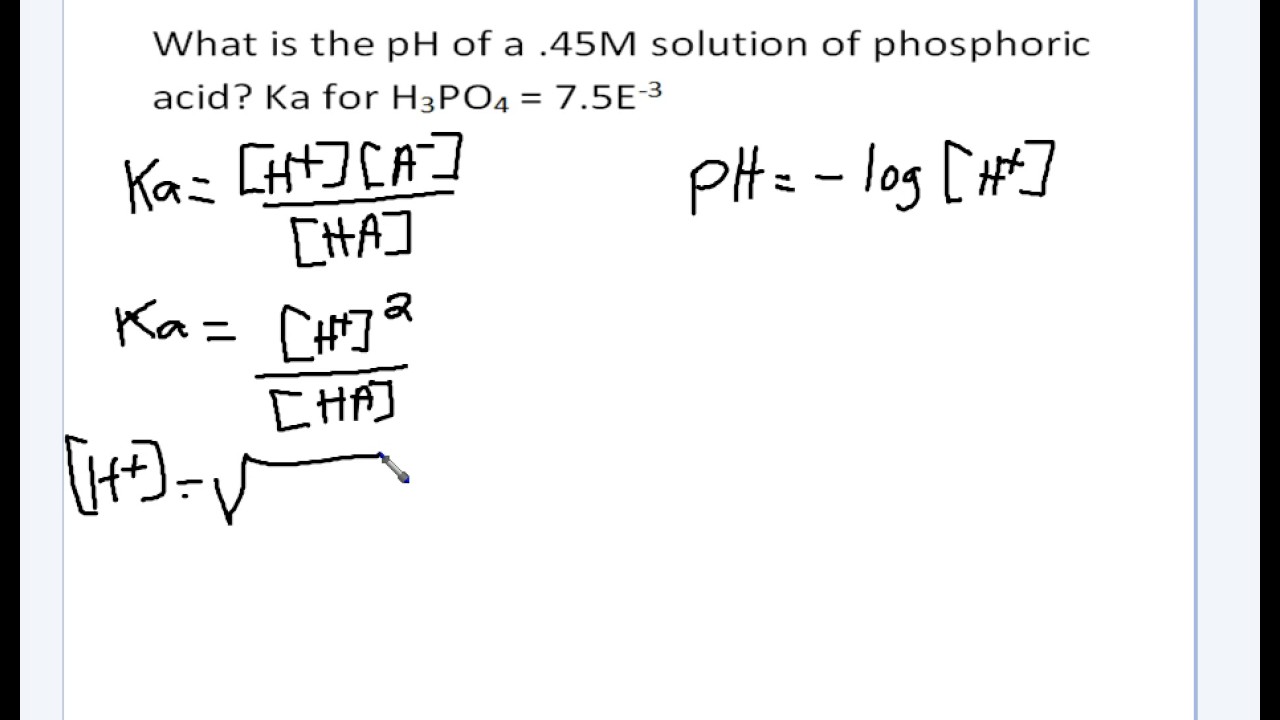

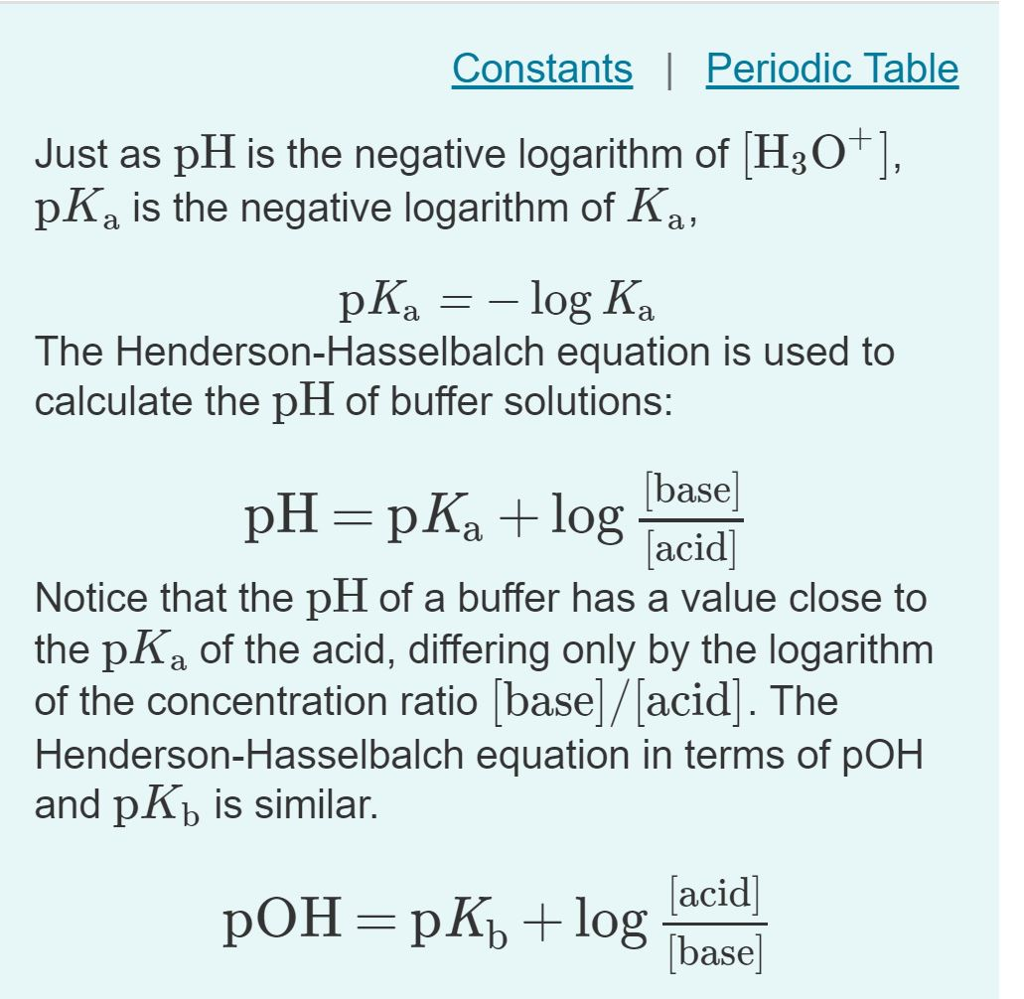


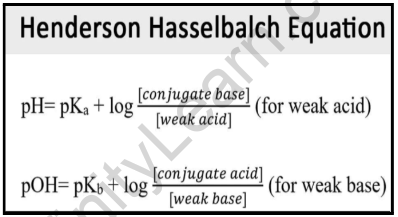


![Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com](https://media.cheggcdn.com/media/375/3755951c-9b2e-4931-a914-33bb100113d8/php72x3Cn.png)





